METRION: The Future of Prostate MRI Calibration
SI-Traceable ADC Measurements for Confident Diagnosis
Proven Performance
When comparing three state-of-the-art 3T MRI scanners with PI-RADS-compliant MRI parameters, biases of up to 25% were observed across these systems. These significant variations were easily corrected using the METRION calibration device.
>25%
Inter-scanner bias correction
Complete System Components
Four integrated components working together to deliver SI-traceable ADC calibration
Co-patient Phantom
Sophisticated foam mattress with embedded SI-traceable reference materials. Features pyrolytic graphite susceptibility matching for distortion-free imaging.
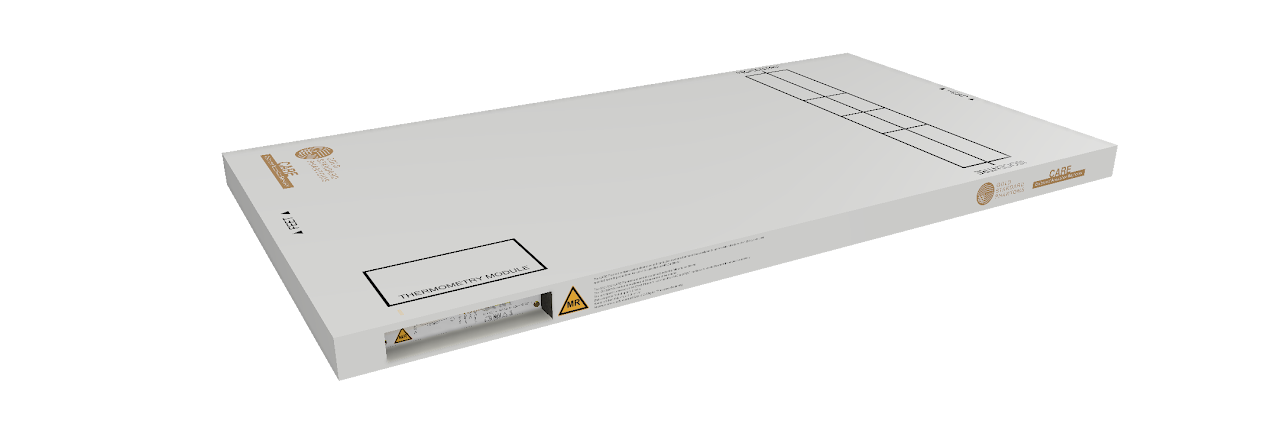
Thermometry Module
MRI-compatible 2-4 fibre optic sensors providing ±0.1°C accuracy. Real-time temperature monitoring throughout the scan.
Docking Station
Central hub for data transfer and charging. Seamless connectivity with hospital networks and PACS systems.
Image Analysis Software
Automated calibration software with PACS integration. Provides instant bias correction and ADC quantification.
The Challenge of Prostate MRI Standardisation
>25%
ADC measurement variability
30%
Indeterminate diagnoses
50%
Unnecessary biopsies
Up to £2,500
Cost per unnecessary biopsy
Current prostate MRI faces significant challenges with reproducibility and standardisation. Even state-of-the-art 3T MRI scanners using PI-RADS-compliant protocols show biases of up to 25% between systems, leading to inconsistent diagnoses and reduced confidence in clinical decisions.
Introducing METRION: Calibrated Confidence
Advanced Phantom Technology
METRION employs cutting-edge co-patient phantom technology with embedded SI-traceable reference materials. The system integrates seamlessly into existing MRI workflows.
- Pyrolytic graphite susceptibility matching for distortion-free imaging
- Multiple reference compartments with NIST-traceable ADC values
- PI-RADS-compliant protocol validation
- Automatic calibration without additional scan sequences
Transforming Prostate Cancer Diagnosis
Clinical applications where METRION makes a measurable difference
Active Surveillance Monitoring
Track ADC changes over time with confidence. Standardised measurements enable reliable detection of disease progression.
Pre-biopsy Assessment
Improve targeting accuracy with quantitative ADC maps. Reduce false positives and unnecessary biopsies.
Treatment Response Evaluation
Monitor therapy effectiveness with precise ADC measurements. Detect changes earlier than conventional imaging.
Multi-centre Clinical Trials
Ensure data consistency across sites and scanners. Enable reliable pooling of quantitative imaging data.
Measurable Impact on Patient Care
Quantitative ADC values with confidence intervals
Eliminate inter-scanner bias of up to 25%
Standardised measurements across all MRI vendors
Automated calibration with PACS integration
Evidence-based diagnostic support
Technical Specifications
Physical Specifications
Thermometry Module
Software Requirements
Frequently Asked Questions
Be Among the First to Experience METRION
Join our waiting list for early access, exclusive launch pricing, and priority support.
Questions? Contact our clinical specialists at info@goldstandardphantoms.com